General
GPC can provide CE certification services through the cooperation of notified bodies. GPC’s nominated auditors can participate in the conformity assessment activities with notified bodies.
The Medical Device Directive is intended to harmonize the laws related to medical devices within the European Union. In order to legally place medical device(s) on the European market, manufacturer(s) should comply with the requirements of the MD Directive. Product(s) and the quality system of an applicant must be assessed and the manufacturer must affix the CE marking before placing the product(s) on the market.
Applicable Products
The Medical Device Directive is applicable to the devices conforming to the definition of "medical device". Medical device? means any instrument, apparatus, appliance, material or other article, whether used alone or in combination, including the software necessary for its proper application intended by the manufacturer to be used for human beings for the purpose of: diagnosis, prevention, monitoring, treatment or alleviation of disease, diagnosis, monitoring, treatment, alleviation of or compensation for an injury or handicap, investigation, replacement or modification of the anatomy or of a physiological process, control of conception, and of which does not achieve its principal intended action in or on the human body by pharmacological, immunological or metabolic means, but which may be assisted in its function by such means.
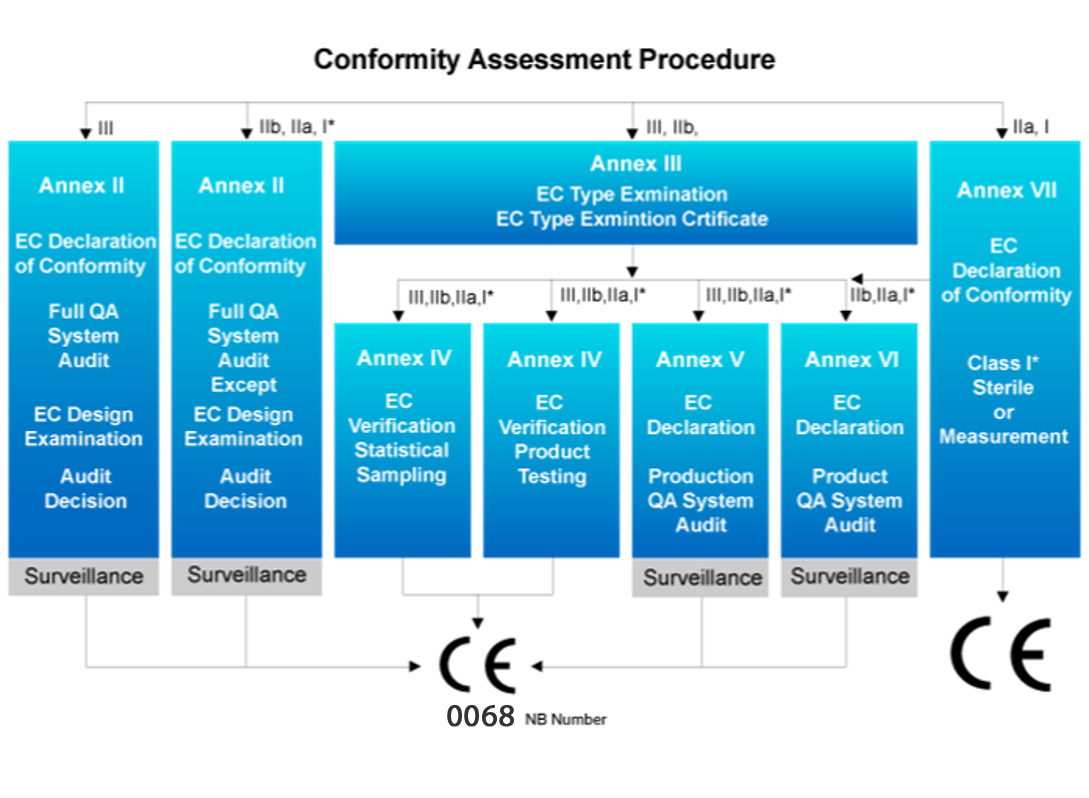
Conformity Assessment Procedure
Classification
Annex IX of Medical Device Directive (93/42/EEC) provides 18 rules to classify medical devices. According to these Rules, medical devices are classified by their intended purposes.
· Rule 1 ~ 4: non invasive devices
· Rule 5 ~ 8: invasive devices
· Rule 9 ~ 12: active devices
· Rule 13 ~ 18: special rules
Risk value of medical devices to patient or user is identified and classified in accordance with those 18 rules.
Depending on the identified risk value, the conformity assessment process is decided.
The Higher the risk value is, the stricter the conformity assessment requirements are.
With the above 18 rules, medical devices are classified into class I, I-measuring, I- sterile, IIa, IIb and III.
1. Class I medical devices follows assessment procedure Annex VII.
2. Procedure of Class I (with measuring function)
· Manufacturer's possible choices:
· Technical documentation according to Sec. 3 of Annex VII and verification of the conformity with metrological requirements on each piece or on a statistically selected sample according to Annex IV
· Technical documentation according to Sec. 3 of Annex VII and assessment of production quality system according to Annex V related to metrological requirements
· Technical documentation according to Sec. 3 of Annex VII and quality assessment of MD according to Annex VI related to metrological requirements
3. Procedure of Class I (with sterile)
· Manufacturer possible choices:
· Technical documentation according to Sec. 3 Annex VII and assessment of production quality system according to Annex V related to sterile conditions
4. Procedure of Class IIa medical devices
· Manufacturer's possible choices:
· Technical documentation according to Sec. 3 of Annex VII and verification of the conformity with the technical documentation on each piece or on a statistically selected sample according to Sec. 8 of Annex IV
· Technical documentation according to Sec. 3 of Annex VII and assessment of production quality system according to Sec. 6 of Annex V (by an audit at the manufacturer's sites)
· Technical documentation according to Sec. 3 of Annex VII and assessment of medical device quality according to Sec. 6 of Annex VI (by an audit at the manufacturer's sites)
· Technical documentation according to Sec. 3 of Annex VII and assessment of full quality system by an audit at the manufacturer's sites according to Annex II (except design examination according to Sec. 4)
5. Procedure of Class IIb medical devices
· Manufacturer's possible choices:
· Technical documentation according to Section 3 of Annex III, examination of type and verification of conformity of each piece manufactured; the verification is done according to Section 5 or on a statistically (randomly) selected sample according to Section 6 of Annex IV
· Technical documentation according to Section 3 of Annex III, examination of type and assessment of production quality system according to Annex V (by an audit of the manufacturer's sites)
· Technical documentation according to Section 3 of Annex III, examination of type and assessment of medical device quality according to Section 6 of Annex VI (by an audit at the manufacturer's sites)
· Technical documentation according to Section 3.2 of Annex II and assessment of full quality system by an audit at the manufacturer's sites according to Annex II (except design examination according to Section 4)
6. Procedure of Class III medical devices
· Manufacturer's possible choices:
· Technical documentation according to Section 3.2 of Annex II, description of the design according to Section 4.2 and assessment of full quality system according to Annex II (by an audit at the manufacturer's sites)
· Technical documentation according to Section 3 of Annex III, examination of type and verification of conformity of each piece produced; the verification is done according to Section 5 or on a statistically (randomly) selected sample according to Section 6 of Annex IV
· Technical documentation according to Section 3 of Annex III and Section 3.2 of Annex V, examination of type and assessment of production quality system (by an audit of the manufacturer's sites)
IRCM's CE/MDD Certification Scopes
According to the compatibility assessment method chosen by the manufacturer (combining the above mentioned annexes) these activities may concern:
- Laboratory test referring to harmonized rules applied to products
- Assessment and similarities to company quality management systems required by directive 93/42/EC.
Istituto Masini has been authorized by the related ministries to operate on the following categories of medical devices:
- Medical devices for anesthesia and reanimation:
- Defibrillation device
- Transcutaneous monitor pO2/pCO2
- Continuous positive pressure, device for manual breathing
- Lung fan
- Continuous flow anesthesia devices.
Medical devices for cardiac surgery
- Extracorporeal circulation heater/cooler
- Sternotome
- Devices for infusion and transfusion
- Devices for plasmapheresis and blood recovery
Medical devices for cardiology
- System for stress test
- External cardiac stimulator
- Cardio-phone
- Bicycle ergometer
- Intra-aortic balloon pump
- Electrocardiograph
- ECG Holter reader
- Runner
- Polygraph
- Holter recorder
- ECG telemetry unit
Medical devices for general surgery
- Nitrogen protoxide analyzer
- Device for anesthesia
- Ultrasound aspiration device
- Medical surgical aspiration device
- Self-transfusion device
- Electric dermatome
- Diathermocoagulator
- Electro-scalpel
- Photocoagulator
- Surgical light
- Surgical laser
- Operating table
- Thermo-welding device
- Optical fibers
- Invasive devices for surgery
Medical devices for physiotherapy and rehabilitation
- Biofeedback device
- Microwave geothermic device
- Shortwave medical diathermy
- Ultrasounds diathermy device
- Electrotherapy device
- Iontophoresis device
- Therapeutic laser
- Intracerebral / subcortical electro-analgesic stimulator
- Spine electro-analgesic stimulator
- Face stimulator
- Muscle stimulator
- Neurological stimulator
- Neuromuscular stimulator
- Bowel washout devices
Gastro-enterology medical devices
- Endoscope
- Light source
- Gas insufflators
- Rectoscope
- Signoidoscope
- TV system for endoscopy
- Camera for endoscopy techniques
Medical devices for gynecology and obstetrics
- Colposcope
- Laparoscope
- Fetal monitor
- Fetal heart rate detector
Medical devices for chemical analysis
- Cutaneous biliruminometer
- Medical devices for general medicine
- Aerosol device
- Emospeedometer
- Phonendoscope
- Device for oncologic hyperthermia
- Hypothermia device
- Infrared lamp
- Ultra-violet/infrared lamp
- Automatic blood pressure meter
- Direct blood pressure meter
- Reaction times meter
- pH monitor
- Nebulizer
- Pump nutrition device
- Ear oximeter
- Plethysmograph
- Infusion pump
- Sphygmomanometer
- Blood pressure monitoring system
- Stethoscope
- Thermometer
- Body thermoregulation device
- Autoclave
- Sterilizers
- Wound bandages and medication
Medical devices for nephrology and blood analysis
- Deionizer
- Device for peritoneal dialysis
- Device for blood-dialysis
- Device for blood filtration
- Balance bed for dialysis
- Heparin pump
- Blood bags
- Bags for tube feeding and parenteral nutrition
- Dialysis filters and concentrates
Medical devices for neurosurgery
- Stereotaxis system
- Neurosurgical drill
Medical devices for nervous system physiopatological neurology
- Doppler spectral analyzer
- EEG spectral analyser
- Echoencelography device
- Electroencelography device
- Electromyography device
- EEG Holter reader
- Device for the analysis of evoked potential
- Electrotherapy stimulators
- EG telemetry unit
- EMG telemetry unit
Medical devices for dentistry
- Dental chairs and delivery units
- Dental tools
- Reconstruction plants
- Tooth plants
- Cements
Medical devices for ophthalmology
- Peripheral vision analyzer
- Device for ophthalmic diathermy
- Electronystagmograph
- Electrooculograph
- Electoretinograph
- Camera for fluorescence angiography
- Diode laser
- Lens meter
- Slit lamp
- Retinoscopy target
- Ophtalmoscopy
- Ophtalmometer
- Ophtalmic lasers
- Ocular tonometry meter
Medical devices for orthopedics and traumatology
- Orthopaedic saw
- Plaster cast stove
- Orthopaedic drill
- Joints for osteosynthesis
Medical devices for otoralingology
- Audimeter
- Acoustic chamber
- Tympanometry
- Laryngoscope
- Nasoscope
- otoscope
- Otoralingology unit
Medical devices for paediatrics and neonatology
- Device for paediatric phototherapy
- Neonatal incubator
- Transport neonatal incubator
- Apnoea monitor
Medical devices for pneumology and physiopathology
- Bronchoscope
- Oxygen concentrator
- Rhinomanometer
- Spirometer
- Bell spirometer
- Dry spirometer
Medical devices for radiology and nuclear medicine
- Bone densitometer
- Dosimeter
- Thermograph
- Magnetic resonance tomographer
- Devices for diagnostic therapy with ionizing radiations
Medical devices for urology
- Cystoscope
- Urodynamics system
- Uroflowmeter
And also
- Contact lenses
- Devices for contact lenses treatment
- Prophylactics
- Bed-heading bars
- Dropping funnels, droppers, drugs dispensers
- Software for the measurement and monitoring of physiological parameters



