ISO 13485
General
ISO 13485 standard specifies requirements for a quality management system that can be used by an organization for the design and development, production, installation and servicing of medical devices, and the design, development, and provision of related services.
While this is a stand-alone standard, it is based on ISO 9001.
The ISO 13485 standard for medical devices – quality management systems – requirements for regulatory purposes, is the basis for regulatory compliance in local and most export markets. Having certification demonstrates your commitment to meeting your customer requirements. ISO 13485 also can be used by internal and external parties, including certification bodies, to assess the organization’s ability to meet customer and regulatory requirements.
Requirements of the ISO 13485 standard
The ISO 13485 standard contains specific requirements for manufacture, installation and servicing and requires
|
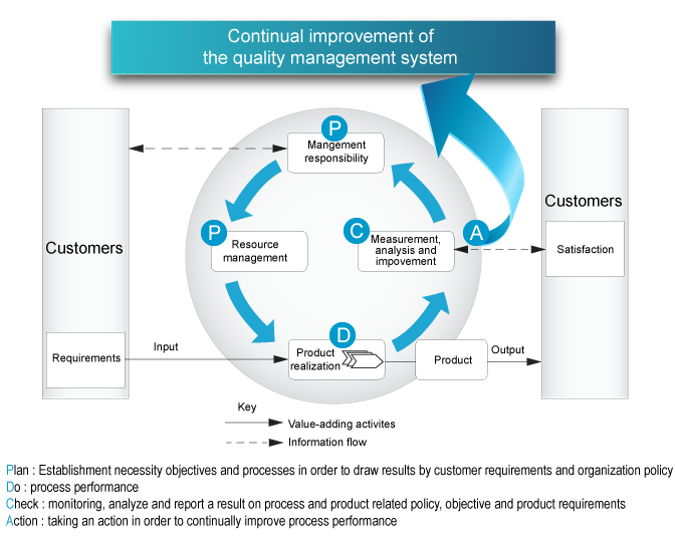
Process Approach
Risk-based Approach
The regulatory audit is risk-based with a focus on key processes of the quality system necessary to manufacture the medical devices. In other words, the auditor should concentrate on
factors that are most likely to affect patient safety. Similarly, it can be considered to depend on the life-cycle, shelf-life, sterilization, raw materials, special processes, active energy, etc. o
f the medical devices.
Essential benefits of ISO 13485
– Meets regulatory requirements
– Demonstrates that medical devices are produced safely
– Increases device sales by accessing more markets.
Further advantages from ISO 13485
- Reduced operating costs
- Proven business credentials
- Improved stakeholder relationships
- Openings in new markets
- Legal compliance
- Customer satisfaction
- Improved risk management